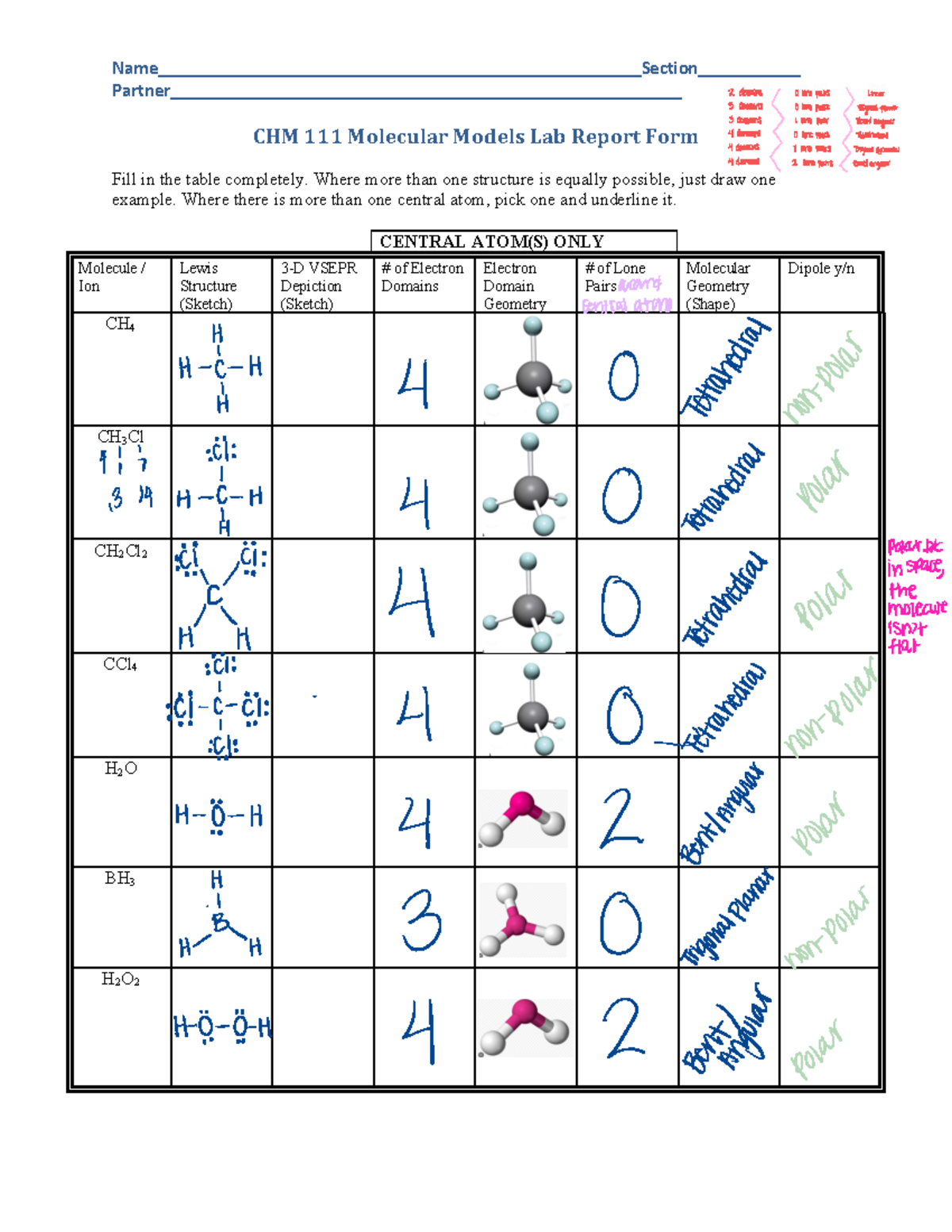
Ap Chemistry Molecular Model Lab Answers . in this experiment you will test properties (melting temperature, solubility in water, and electrical conductivity of water solution) of substances. there are ap style practice problems that should give you feedback as you work. the purpose of this lab is to use molecular models to identify each substance, and to practice applying principles of lewis. To practice drawing lewis dot structures, to apply vsepr theory to predict molecular. write lewis structure representations of the bonding and valence electrons inmolecules. Use the vsepr model to predict the. I will give you a completion grade for each one if you complete them. The bond angles actually present in a molecule of methane are all approximately 109.5o.
from www.studocu.com
there are ap style practice problems that should give you feedback as you work. in this experiment you will test properties (melting temperature, solubility in water, and electrical conductivity of water solution) of substances. I will give you a completion grade for each one if you complete them. Use the vsepr model to predict the. write lewis structure representations of the bonding and valence electrons inmolecules. the purpose of this lab is to use molecular models to identify each substance, and to practice applying principles of lewis. To practice drawing lewis dot structures, to apply vsepr theory to predict molecular. The bond angles actually present in a molecule of methane are all approximately 109.5o.
111Lab Molecular Models RF+v2021 Studocu
Ap Chemistry Molecular Model Lab Answers The bond angles actually present in a molecule of methane are all approximately 109.5o. there are ap style practice problems that should give you feedback as you work. I will give you a completion grade for each one if you complete them. Use the vsepr model to predict the. write lewis structure representations of the bonding and valence electrons inmolecules. To practice drawing lewis dot structures, to apply vsepr theory to predict molecular. The bond angles actually present in a molecule of methane are all approximately 109.5o. the purpose of this lab is to use molecular models to identify each substance, and to practice applying principles of lewis. in this experiment you will test properties (melting temperature, solubility in water, and electrical conductivity of water solution) of substances.
From paytenghopramos.blogspot.com
Molecular Modeling and Lewis Structures Lab Answers Ap Chemistry Molecular Model Lab Answers in this experiment you will test properties (melting temperature, solubility in water, and electrical conductivity of water solution) of substances. there are ap style practice problems that should give you feedback as you work. Use the vsepr model to predict the. The bond angles actually present in a molecule of methane are all approximately 109.5o. To practice drawing. Ap Chemistry Molecular Model Lab Answers.
From www.studypool.com
SOLUTION Molecular shapes and dipole chart study note ap chemistry Ap Chemistry Molecular Model Lab Answers write lewis structure representations of the bonding and valence electrons inmolecules. I will give you a completion grade for each one if you complete them. in this experiment you will test properties (melting temperature, solubility in water, and electrical conductivity of water solution) of substances. the purpose of this lab is to use molecular models to identify. Ap Chemistry Molecular Model Lab Answers.
From www.studocu.com
Molecular Geometry Pre Lab CHEM 201 Molecular Geometry and Bonding Ap Chemistry Molecular Model Lab Answers I will give you a completion grade for each one if you complete them. To practice drawing lewis dot structures, to apply vsepr theory to predict molecular. Use the vsepr model to predict the. there are ap style practice problems that should give you feedback as you work. The bond angles actually present in a molecule of methane are. Ap Chemistry Molecular Model Lab Answers.
From www.pinterest.ca
Building Molecular Models Lab November 20, 2017 Molecular Ap Chemistry Molecular Model Lab Answers Use the vsepr model to predict the. I will give you a completion grade for each one if you complete them. there are ap style practice problems that should give you feedback as you work. The bond angles actually present in a molecule of methane are all approximately 109.5o. To practice drawing lewis dot structures, to apply vsepr theory. Ap Chemistry Molecular Model Lab Answers.
From exoapjzaz.blob.core.windows.net
Lab 10 Questions For Molecular Models at Ted Winger blog Ap Chemistry Molecular Model Lab Answers write lewis structure representations of the bonding and valence electrons inmolecules. To practice drawing lewis dot structures, to apply vsepr theory to predict molecular. there are ap style practice problems that should give you feedback as you work. in this experiment you will test properties (melting temperature, solubility in water, and electrical conductivity of water solution) of. Ap Chemistry Molecular Model Lab Answers.
From www.coursehero.com
[Solved] Lab 10 Molecular Geometry Lab Lewis Structure Molecular Ap Chemistry Molecular Model Lab Answers Use the vsepr model to predict the. To practice drawing lewis dot structures, to apply vsepr theory to predict molecular. there are ap style practice problems that should give you feedback as you work. write lewis structure representations of the bonding and valence electrons inmolecules. The bond angles actually present in a molecule of methane are all approximately. Ap Chemistry Molecular Model Lab Answers.
From www.chegg.com
Solved MOLECULAR MODELING LAB. Use the key below Ap Chemistry Molecular Model Lab Answers there are ap style practice problems that should give you feedback as you work. in this experiment you will test properties (melting temperature, solubility in water, and electrical conductivity of water solution) of substances. Use the vsepr model to predict the. write lewis structure representations of the bonding and valence electrons inmolecules. The bond angles actually present. Ap Chemistry Molecular Model Lab Answers.
From sarikatt.blogspot.com
Sarika's AP Chem Blog! Week 5 Blog Entry Ap Chemistry Molecular Model Lab Answers in this experiment you will test properties (melting temperature, solubility in water, and electrical conductivity of water solution) of substances. the purpose of this lab is to use molecular models to identify each substance, and to practice applying principles of lewis. To practice drawing lewis dot structures, to apply vsepr theory to predict molecular. Use the vsepr model. Ap Chemistry Molecular Model Lab Answers.
From studylib.net
AP Chemistry Molecular Geometry Ap Chemistry Molecular Model Lab Answers To practice drawing lewis dot structures, to apply vsepr theory to predict molecular. I will give you a completion grade for each one if you complete them. in this experiment you will test properties (melting temperature, solubility in water, and electrical conductivity of water solution) of substances. the purpose of this lab is to use molecular models to. Ap Chemistry Molecular Model Lab Answers.
From printableschoolenvenoms.z13.web.core.windows.net
Ap Chemistry Molecular Geometry Worksheets Ap Chemistry Molecular Model Lab Answers in this experiment you will test properties (melting temperature, solubility in water, and electrical conductivity of water solution) of substances. To practice drawing lewis dot structures, to apply vsepr theory to predict molecular. The bond angles actually present in a molecule of methane are all approximately 109.5o. Use the vsepr model to predict the. there are ap style. Ap Chemistry Molecular Model Lab Answers.
From www.studocu.com
Chem 236 Molecular Lab Final Molecular Modeling Report Sheet (37 pts Ap Chemistry Molecular Model Lab Answers there are ap style practice problems that should give you feedback as you work. I will give you a completion grade for each one if you complete them. in this experiment you will test properties (melting temperature, solubility in water, and electrical conductivity of water solution) of substances. the purpose of this lab is to use molecular. Ap Chemistry Molecular Model Lab Answers.
From www.coursehero.com
[Solved] complete Molecular Models Lab Compounds and Other Information Ap Chemistry Molecular Model Lab Answers I will give you a completion grade for each one if you complete them. The bond angles actually present in a molecule of methane are all approximately 109.5o. the purpose of this lab is to use molecular models to identify each substance, and to practice applying principles of lewis. To practice drawing lewis dot structures, to apply vsepr theory. Ap Chemistry Molecular Model Lab Answers.
From www.studocu.com
111Lab Molecular Models RF+v2021 Studocu Ap Chemistry Molecular Model Lab Answers the purpose of this lab is to use molecular models to identify each substance, and to practice applying principles of lewis. write lewis structure representations of the bonding and valence electrons inmolecules. To practice drawing lewis dot structures, to apply vsepr theory to predict molecular. there are ap style practice problems that should give you feedback as. Ap Chemistry Molecular Model Lab Answers.
From www.chegg.com
Solved Lab 7 Molecular Models Section Date Postlab Questions Ap Chemistry Molecular Model Lab Answers the purpose of this lab is to use molecular models to identify each substance, and to practice applying principles of lewis. write lewis structure representations of the bonding and valence electrons inmolecules. in this experiment you will test properties (melting temperature, solubility in water, and electrical conductivity of water solution) of substances. there are ap style. Ap Chemistry Molecular Model Lab Answers.
From www.studocu.com
Molecular Models Lab Name __________________________ Study Ap Chemistry Molecular Model Lab Answers To practice drawing lewis dot structures, to apply vsepr theory to predict molecular. The bond angles actually present in a molecule of methane are all approximately 109.5o. there are ap style practice problems that should give you feedback as you work. Use the vsepr model to predict the. in this experiment you will test properties (melting temperature, solubility. Ap Chemistry Molecular Model Lab Answers.
From answerfullallan101.z19.web.core.windows.net
Molecular Shapes Worksheet Answers Ap Chemistry Molecular Model Lab Answers To practice drawing lewis dot structures, to apply vsepr theory to predict molecular. I will give you a completion grade for each one if you complete them. write lewis structure representations of the bonding and valence electrons inmolecules. The bond angles actually present in a molecule of methane are all approximately 109.5o. there are ap style practice problems. Ap Chemistry Molecular Model Lab Answers.
From answerfullallan101.z19.web.core.windows.net
Molecular Shapes Simulation Worksheet Answers Ap Chemistry Molecular Model Lab Answers The bond angles actually present in a molecule of methane are all approximately 109.5o. there are ap style practice problems that should give you feedback as you work. in this experiment you will test properties (melting temperature, solubility in water, and electrical conductivity of water solution) of substances. the purpose of this lab is to use molecular. Ap Chemistry Molecular Model Lab Answers.
From www.chegg.com
Solved Lab 5 Molecular Models Concepts to explore Ap Chemistry Molecular Model Lab Answers The bond angles actually present in a molecule of methane are all approximately 109.5o. there are ap style practice problems that should give you feedback as you work. To practice drawing lewis dot structures, to apply vsepr theory to predict molecular. Use the vsepr model to predict the. in this experiment you will test properties (melting temperature, solubility. Ap Chemistry Molecular Model Lab Answers.